Introduction: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare blood disorder characterized by complement-mediated hemolytic anemia. Terminal complement component C5 inhibitors eculizumab (ECU) and ravulizumab (RAVU) inhibit the formation of membrane attack complex, and thereby ameliorates intravascular hemolysis in PNH. Some patients exhibit breakthrough intravascular hemolysis near the end of 14-day treatment cycle for ECU or 8-week treatment cycle for RAVU. To manage breakthrough hemolysis, different treatment strategies may be attempted in the clinical practice, including shortening the dosing interval and/or increasing the dose of ECU and RAVU. This study aimed to estimate the incremental costs associated with dose escalation in patients with PNH treated with ECU or RAVU in the US.
Methods: Adult (age ≥18 years) patients with PNH (ICD-10-CM code D59.5) treated with ECU or RAVU from health plan claims data (01/01/2011 to 09/30/2022) with ≥3 months of continuous health plan coverage following the first claim for ECU or RAVU were included in this retrospective cohort study. Dosing patterns were investigated during the maintenance phase. Consistent with label-recommended dosing schedule, the maintenance phase started from day 29 and day 15 post-first claim for ECU and RAVU, respectively, until the earliest of treatment discontinuation/loss to follow-up/end of data. Dose escalation for ECU was defined as 1) an increased dose (i.e., 1,200 or 1,500 mg) and/or 2) an increased infusion frequency (i.e., ≥2 infusions within 12 days). Patient-weight information was not available; dose escalation for RAVU was defined as an increased infusion frequency (i.e., ≥2 infusions within 49 days). All-cause and PNH-related direct healthcare costs measured during maintenance phase for the overall cohort and subgroup of patients with dose escalation were assessed for ECU and RAVU separately. Incremental costs associated with dose escalation in the pre vs. post dose escalation period were assessed for ECU and RAVU separately; p-values from Wilcoxon signed-rank tests were reported.
Results: A total of 278 patients treated with ECU (mean± SD age, 42±13 years; 60% female) were included in the overall cohort and a subgroup of 151 patients were observed with a dose escalation (mean±SD age, 41±13 years; 58% female). For the overall ECU cohort (N=278), during a mean follow-up of 23 months, total all-cause and PNH-related costs (mean±SD) were $67,650±$32,518 and $65,751±$31,379 per patient per month (PPPM), respectively. In the subgroup of patients with dose escalation (N=151), over a mean follow-up of 30 months, total all-cause and PNH-related costs (mean±SD) were $71,466±$33,452 and $69,471±$31,595 PPPM, respectively. In the overall cohort and subgroup of patients with dose escalation, PNH-related treatment costs represented 90% and 92% of total all-cause costs respectively.
A total of 171 patients treated with RAVU (mean± SD age, 39±12 years; 43% female) were included in the overall cohort and a subgroup of 26 patients were observed with a dose escalation (i.e., increased infusion frequency only) (mean± SD age, 37±11 years; 54% female). For the overall RAVU cohort (N=171), during a mean follow-up of 17 months, total all-cause and PNH-related costs (mean±SD) were $50,779±$26,258 and $49,213±$25,751 PPPM, respectively. In the subgroup of patients with dose escalation (N=26), over a mean follow-up of 20 months, total all-cause and PNH-related costs (mean±SD) were $52,819±$20,965 and $50,740±$20,837 PPPM, respectively. In the overall cohort and subgroup of patients with dose escalation, PNH-related treatment costs represented 93% and 91% of total all-cause costs, respectively.
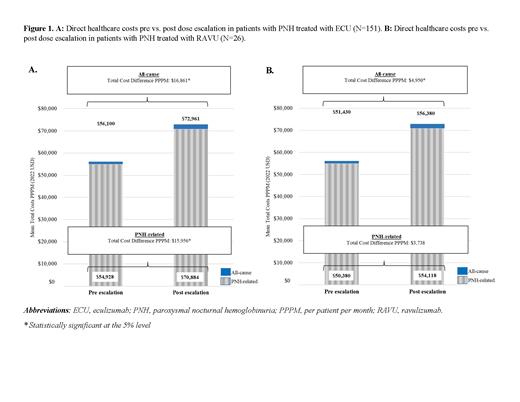
Incremental costs (mean cost differences) associated with dose escalation for ECU were of $16,861 (p<0.01) and $15,956 (p<0.01) PPPM for all-cause and PNH-related total costs, respectively; and for RAVU were of $4,950 (p<0.05) and $3,738 (p=0.06) PPPM for all-cause and PNH-related total costs, respectively (Figures 1A and 1B).
Conclusions: In this study of PNH patients treated with C5 inhibitors, dose escalation was associated with significant incremental costs for both ECU and RAVU. Further research is warranted to clarify the reasons for dose escalation, which may potentially indicate an unmet clinical need for more efficacious treatment options to help prevent breakthrough symptoms and reduce the burden of PNH.
Disclosures
Tantravahi:Partnership for Health Analytic Research LLC: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; CTI BioPharma: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Karyopharm Therapeutics Inc: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria. Latremouille-Viau:Bristol Myers Squibb: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Pfizer Inc.: Consultancy, Research Funding; Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Desai:Novartis Pharmaceuticals Corporation; Takeda Pharmaceuticals USA, Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Lee:Novartis Pharmaceutical Corporation: Current Employment. Paulose:Novartis Pharmaceuticals Corporation: Current Employment. Geevarghese:Novartis Pharmaceuticals Corporation: Current Employment. Guérin:Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Analysis Group: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; AbbVie: Consultancy. Seshasayee:Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Tabatabaeepour:Analysis Group, Inc.: Current Employment, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.. Yen:Novartis Pharmaceuticals Corporation: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal